 |
| Posing with a pseudostalagmite, Oman, January 2009. |
def. Speleothem:
An encompassing term used to describe all types of chemical precipitates that form in caves.
If you’ve ever been in a cave, you’ve probably seen speleothems. Speleothems generally precipitate from groundwater which has percolated through the bedrock surrounding the cave and leached various elements and compounds. When the enriched water reaches the cave, changing conditions (a large open space has very different pressure and temperature than pore spaces in bedrock) allow gases, such as carbon dioxide, to escape from the water. Evaporation can also occur. The changing composition of the water encourages the (usually very, very slow) precipitation of speleothem minerals from cave waters.
Chemically, the speleothems which form in a particular cave are similar in composition. Most caves are formed in limestone, and so the speleothems will generally be formed of calcite, the dominant mineral in limestone. However, depending on how and where the speleothem is precipitated, it can take on a variety of shapes. Scientists and other cave explorers have given different names to these various morphologies. Examples of speleothems are stalactites, stalagmites, flowstones, cave coral, cave drapery, cave curtains, and cave crystals. There are dozens of names for various cave mineral formations, so speleothem is a nice catch-all phrase for geologists to use.
Here is a great picture (from Wikipedia) of some of the most common types of speleothems:
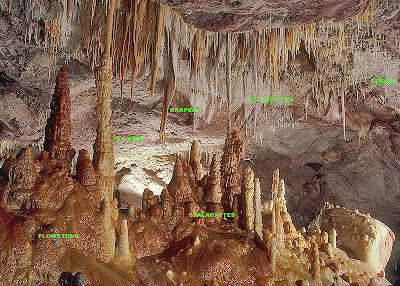 |
| Photo by Dave Bunnell of some common speleothems. Taken from Wikipedia here. Click photo to enlarge. |
Most speleothems form over thousands upon thousands of years. Thus, you shouldn’t touch or remove speleothems unless you’re doing so for legitimate science. Even when collecting speleothems for science, one should be conservative. Geologists should take small samples and obtain the necessary permissions. Fortunately, for my own research in Oman I am often able to collect speleothems which have fallen on the ground and are no longer growing.
At the top of this post is a picture of me with a stalagmite in Oman. I didn’t sample this one, but I did sample some of its neighbors. This particular stalagmite isn’t forming in a true cave but rather in an open hallow underneath a layer of rock. Water percolated through the layer of rock and formed speleothems in the hallow underneath. The speleothems I study in Oman are thus really pseudospeleothems– they are not in true caves but rather in little overhangs and hallows.
Now, for those of you who still confuse stalactite and stalagmite, here’s a reminder of something you probably learned in grammar school but may have forgotten by now:
StalaCtites hang tight (or tite) to the Ceiling while stalaGmites grow up from the Ground.
Finally, below are few more pictures of some Omani pseudospeleothems. These pseduospeleothems are forming in overhangs in travertines (carbonate precipitates) which are forming at the surface of the peridotite layer of the ophiolite. Be sure to click on the two panoramas to enlarge them. Note the location of the pseudospeleothem column in the two panoramas. Many pseudospeleothems are located in the overhang around this column. The last picture has my colleague Lisa standing next to this column for scale; this shows the enormous size of the travertine deposit.
 |
| Travertine pseudospeleothems, Oman, January 2009. |
 |
| Water dripping from a pseduospeleothem straw, Oman, January 2009. |
 |
| Panorama of Wadi Sudari Travertine I, Oman, January 2010. |
 |
| Panorama of Wadi Sudari Travertine II, Oman, January 2010. |
 |
| Lisa standing next to an enormous column of travertine, Oman, January 2009. |

















