 |
| Elephant in the Rotunda, Smithsonian Museum of Natural History. Picture taken from wikipedia here. |
There is nothing better than having an empty museum all to yourself. If I ever become wealthy, I think that I’ll rent out the great museums of the world at odd hours so that I can quietly appreciate them. Or maybe I’ll just hone my burglary skills and sneak it at night. I’m not interested in stealing anything, just enjoying the richness of the exhibits without all the lines, tourists, and misbehaving toddlers.
One of my favorite books from childhood is From the Mixed-up Files of Mrs. Basil E. Frankweiler, a book about a little girl and her brother who run away from home and live in the Metropolitan Museum of Art for a week or so. After all of the tourists go home for the day, the two children have the run of the museum, bathing in the public fountain, sleeping in an antique bed in one of the exhibits, and generally having a good time with all of the artwork and artifacts. After reading this book, I nearly packed a bag and ran away to the nearest big museum, which fortunately was several hours away. Running away from home is not easily accomplished by an eight-year-old stuck in rural New Hampshire with no public transportation.
Just over a year ago [Note: in 2005 since this is a re-post], I was again ready to run away from home (well, from my apartment, I guess…) and move into a museum. More specifically, I was ready to move into the Smithsonian Department of Mineral Sciences, the behind-the-scenes home of the thousands of rocks, minerals, and gems which are not on public display. Far less than 1% of the Smithsonians mineral collection is on display for the public. The reason there is so little on display relates to space constraints, not to lack of impressive specimens.
There are hundreds upon hundreds of more minerals and gems that should be on public display but which are instead tucked away in storage. Yes, I’m quite willing to take sponge baths in the department bathroom and roll out my sleeping bag in one of the labs or collection rooms. So many beautiful samples to appreciate, so little time. I really must move in there.
As part of a summer internship in geochemistry, I went to the Smithsonian Natural History Museum to pick up some volcanic glass samples. Really, the samples could have been Fed-Exed to the lab where I was working, but I was so excited about the prospect of interacting with the curators of the Smithsonian that my summer boss surprised me with a ticket to Washington and meetings with several of the curators.
At couple of weeks later, I flew up to Washington and took the subway to the museum. I went in the public entrance and found the special office where I was signed in as a visitor. Tim, a researcher in the volcanology and petrology division, came down and brought me up to the department. I felt very cool and professional as I was led upstairs– not everyone gets to go behind-the-scenes at the Smithsonian! I was also very curious. Tim led me by doors that had very interesting signs, such as “meteorite collection” and “time-of-flight mass spectrometer” and by laboratories that had intriguing-looking machines and chemicals. I didn’t realize that the Smithsonian did so much research. In addition to taking care of the thousands upon thousands of mineral, gem, and rock samples in their collection, they also actively conduct research on many of the samples.
Within about five minutes of arriving at Tim’s office, my “work” for going up to the Smithsonian was done. Tim had already organized, photographed, and documented the samples I was taking, so all he had to do was hand me the envelope, really. There was an awkward moment or two, then finally I asked somewhat sheepishly and also as politely as possible, “So, could I please see some of your rocks?”
Tim generously indulged my interest, showing me bits and pieces of the volcanological reference collection, the seafloor collection, and the ultramafic xenolith collection. We chatted about rocks and petrology as he browsed for interesting samples. I asked him if there were any fulgurites in the collection, and he showed me several. I’d never seen a fulgurite before, and I was impressed.
After showing me through the rock collections, Tim arranged for me to be shown some of the mineral, gem, and meteorite collections. For the rest of the afternoon, I was given attention by various middle-aged, male curators who clearly don’t see too many pretty, young females in their day-to-day work. They easily impressed me. The mineral collection took my breath away. There were a few particularly impressive samples on display in large glass cases, and the rest were neatly filed away in dozens upon dozens of large, metal cabinets. The curator of the mineral collection knew the place by heart and was a walking encyclopdia of mineral knowledge. He kept trying to find unusual specimens to impress me. At one point, he told me to close my eyes and hold out my hands. He filled my hands with what felt like many crystals, maybe a half-inch in diameter. After a few seconds, my hands become very cold. I felt as if the crystals were sucking all the heat out of my hands. The mineral curator told me to open my eyes, and when I did I saw that there were about two dozen large, raw diamonds in my hands. A single one of those diamond crystals would probably have paid for my college education…
I was able to see only a couple of pieces in the gem collection, which has somewhat tighter security than the rest of the collection. However, who needs gems when you can see meteorites? The meteorite samples are kept in a large, room-sized vault. Another Tim, whom I’ll refer to as “Meteorite Tim,” showed me the collection. There were many impressive samples, but I think I enjoyed looking at the tektites most. Filed away with the tektites were several samples of Libyan Desert Glass, a beautiful yellow-green glass that is actually impact glass created when a meteorite hit the desert sands millions of years ago.
I became so interested in this glass that I wrote an article about it for Skeptic Report shortly after my trip to the Smithsonian. I’m pleased to say that Dr. Farouk al-Baz recently discovered Kabira Crater, the crater formed by the meteorite that almost certainly formed this desert glass. Note that I made a mistake in this article– a small one, but one I think I should point out. Cristobalite is a high-temperature polymorph of quartz, not a high-pressure one. The high-pressure polymorph of quartz is actually sitshovite. You can find both cristobalite and stishovite in the desert glass, I believe, but I’d double-check with Meteorite Tim on that.
Anyway, after showing me many wonderful tektites and meteorites, Meteorite Tim pointed to a very special-looking meteorite sitting in a fancy, round glass case.
“Do you know what that is?” he asked.
I didn’t have a clue. I peered at the rock and guessed, “A moon rock?”
Meteorite Tim picked up the sample, case and all, and put it under a nearby microscope.
“Look there,” he said.
I looked at the microscope, adjusting the focus slightly and trying to look all professional. I saw a few reddish-brown little patches on the rock surface. I still had no idea what I was looking at.
Finally, Meteorite Tim said, “This is ALH 84001.”
I jumped, saying, “Really?” I eagerly peered back through the microscope.
“Is that…?” I couldn’t even finish my question.
“Yes, that’s the little bit of rock that caused the big ‘life on Mars’ debate.”
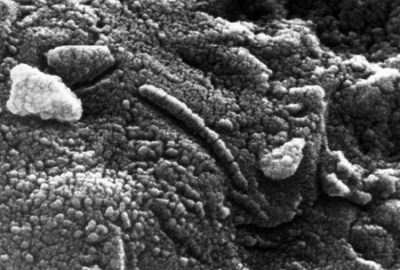 |
| Microscopic view of ALH84001. Image taken from wikipedia here. |
I didn’t see a view of the meteorite that was this close-up, but the above picture gives you a sense of what I was looking at… possible traces of fossil life from another planet. Now, that’s a cool rock.
Honestly, I think that the recent images from Mars showing evidence that water is actively transforming the planet are far more compelling evidence for the potential for life on Mars than some possible little fossilized traces of life in a single Mars meteorite. Still, seeing a rock from Mars– any rock from Mars, since there’s only thirty or so identified Mars rocks here on Earth– is a neat thing to see. I was definitely ready to move into the meteorite vault at that point.
Finally, after several hours of showing me around, I decided that I had taken up enough time of the generous curators and scientists in the Mineral Science Department. I was impressed with how they treated me. I was just an undergraduate student at the time, but they treated me with great respect. Of course, they tried to impress me by showing off their best samples, but in their conversation they engaged me as if I were a colleague of theirs. In a way, I guess I was. After all, I was taking some of their precious rocks away with me.
I think that this story highlights one of the biggest perks of being a scientific researcher: access to all kinds of neat museums and the knowledgeable researchers and curators who work there. Scientists maybe don’t get paid a lot, but who would begrudge, say, The Bad Astronomer a behind-the-scenes tour of a meteorite collection? Maybe my friends with consulting and finance jobs make far more money than I ever will as a scientist, but how many behind-the-scenes tours of the Smithsonian’s Mineral Department have they had? Yes, there are definitely perks to being a scientist.
At the end of my afternoon visit, I walked out the front door of the Smithsonian with three dozen volcanic glass samples in my purse. Security didn’t even check my bag… I should have palmed one of those diamonds. Or, better yet, a meteorite.






